August 2022
“States of matter” just means what state, or form, some matter is in. Water can be ice (a solid), regular water (a liquid), or steam (a gas). These are three “states” of water. Even though the water looks and acts differently as a solid, liquid, and gas, it is still water—that is, the state or form of the water changes but the water itself does not change. Water in different forms—ice, liquid, or steam—is still just water.
When atoms join or group together, we say they “are bonded” or “have formed bonds”. Three types of bonds are important when talking about states of matter. They are covalent, ionic, and metallic bonds.
When atoms bond together by sharing electrons, the bond is called “covalent”.
When atoms bond together because one is negatively charged and the other is positively charged, the bond is called “ionic”.
“Metallic” bonds are different (and a bit more complicated) but also involve both electrons and positive and negative charges. “Metallic” bonding gives metals many of their important properties, such as high conductivity.
How well electricity moves through something is called conductivity. Copper is used for wires because electricity moves through it very easily—copper has high conductivity. Rubber or plastic is often used to insulate the copper wires because electricity does not move easily through rubber or plastic—they have low conductivity.
How much mass there is of something in a given volume is called density. Something with more mass in less space is dense, or has high density. Think of a small but heavy metal ball. Something with less mass in the same (or more) space is not dense, or has low density. Think of piece of paper crumbled up to be the same size as the metal ball. The would be the same size, but the metal ball would be much heavier.
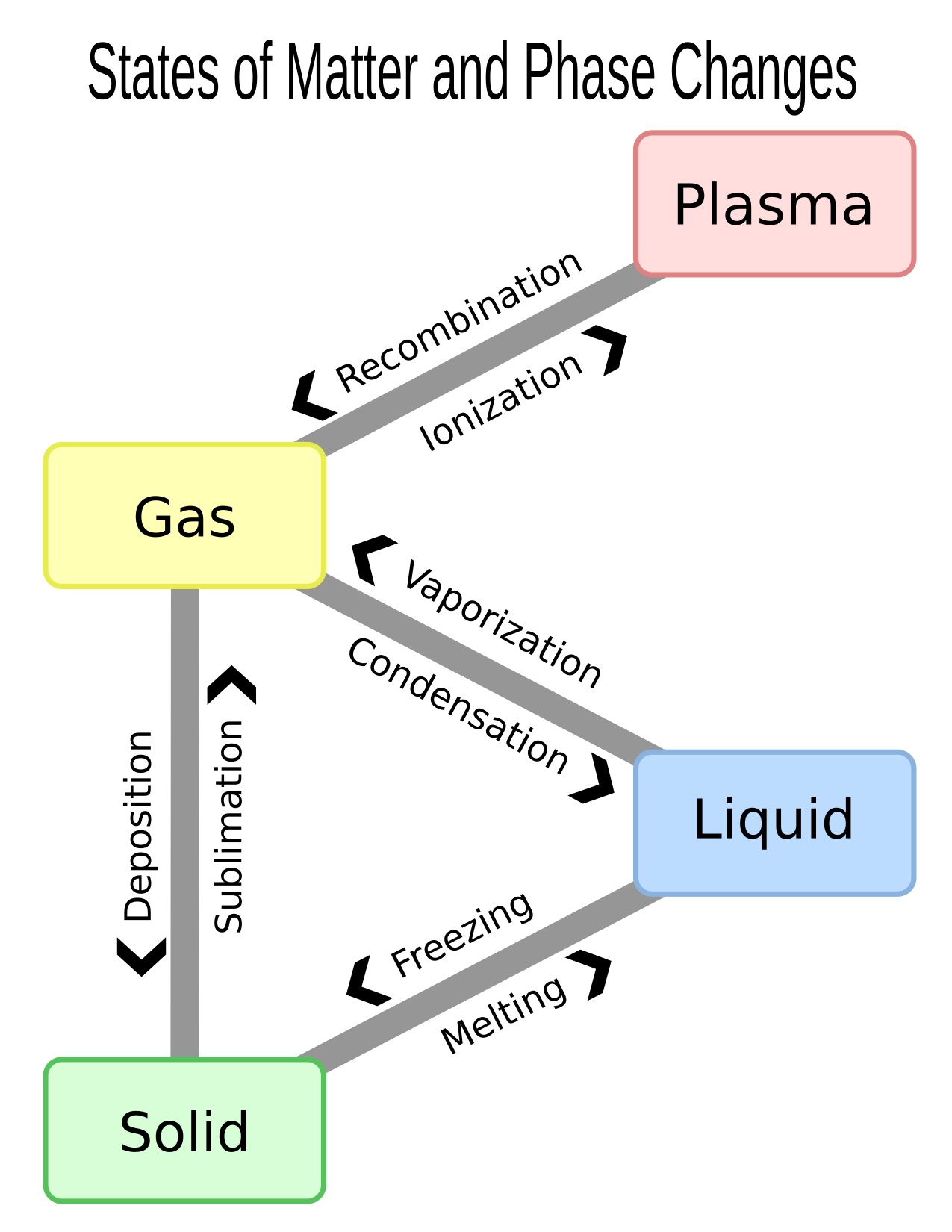
There are four common states of matter (sometimes called “phases of matter”): solid, liquid, gas, and plasma. The state of matter affects a substance’s properties, such as density, viscosity (how well it flows), malleability (how easy it is to bend), and conductivity.
In a solid, atoms form a structure and stay in position for a long time—together, they hold a shape and do not easily change to a different shape. This is because the atoms and molecules “stick” together and do not move easily. This cohesion1 is provided by metallic, covalent, or ionic bonds. If you push on a solid, it normally will not change shape. We say that solids are resistant to deformation. That just means that they don’t change shape easily. The atoms are strongly held in position. Solids keep their own shape, with or without a container.
In solids, the atoms do not have enough energy to escape from the bonds. This is why adding heat to solids melts them—the heat gives the atoms more energy. Eventually, the atoms have enough energy that they break free from the bonds.

Most materials are more dense as solids than they are as liquids. Water is an important exception—ice is less dense than liquid water, which is why ice floats in water.2
When a solid turns into a liquid, we say the solid is “melting”. When a solid becomes a gas without turning into a liquid first the solid is “sublimating”. You might see this in the winter when ice on the sidewalk disappears without ever leaving a puddle or other liquid water behind.
In a liquid, molecules are attracted to other molecules strongly enough to keep them in contact, but not strongly enough to form a structure (that is, not strongly enough to turn the material into a solid). The molecules can continually move around each other. This means that liquids can move around smoothly. However, they do not move as smoothly as gases do.
Usually, liquids take the shape of a container that they are in. The shape of liquids is easily changed by outside forces. Most materials are less dense as liquids than they are as solids. This means that solids will sink to the bottom if put in with the liquid of the same material. For example, solid iron will not float on top of liquid iron because the solid iron is denser than liquid iron. 3
When a liquid turns into a solid, we say the liquid is “freezing” or “solidifying”. The difference is just which state is more “normal” for the material at room temperature. Water is a liquid at room temperature, so we say that it is “freezing” when it turns solid. Iron is a solid at room temperature, so we say liquid iron is “solidifying” when it turns into a solid. When a liquid turns into a gas, it is “vaporizing”.
In a gas, the atoms have so much evergy that chemical bonds are not strong enough to hold atoms or molecules together in either liquid or solid form. Each atom or molecule has a lot of energy. They will not stick together long enough for a liquid or a solid to form. Gases are collections of independent, unbonded molecules which interact mainly by collision4. Gases tend to take the shape of their container. Gases are less dense than both solids and liquids.
When a gas turns into a liquid, we say it is “condensing”. When a gas turns into a solid without turning into a liquid first, we say the gas is “depositing”.
Plasmas are gases that have a lot of energy. They have so much energy that the electrons cannot stay in orbit. After the atoms lose their electrons, the atom has a positive charge, and is an “ion”. The ions and the free electrons mix around like a hot soup.5
Because the positive and negative charged particles are not stuck together, plasma is a good conductor of electricity. For example, air is not good at conducting electricity. However, in a bolt of lightning, the atoms in air get so much energy that they can not hold on to their electrons. For a very short time, the air turns into plasma. Then, electrical current is able to flow through the plasma, making the lightning.
Plasma is the most common state of matter in the universe. This is because stars are mostly made of plasma.
Pressure and temperature can both cause changes in the state of something.6 Temperature measures the amount of energy that molecules in a substance have. The state of a substance depends on how much energy its atoms or molecules have. So, it makes sense that if we add lots of energy, the temperature will go up. When that happens, the state of the substance might change. The name for the temperature at which something changes from a solid to a liquid is called the “melting point”.
The temperature at which something changes from a liquid to a solid is called the “freezing point”. The freezing point and the melting point are the same for a substance, because the only difference is which direction the substance is going. Any increase in temperature will cause it to melt and any drop in temperature will cause it to freeze. For the same reason, the “vaporizing point” (going from liquid to gas) and “condensation point” (going from gas to liquid) are the same.
This reading was adapted from the content available at the links below.
at Simple Wikipedia. Accessed 2022 August 15. Published under the Creative Commons Attribution-ShareAlike 3.0 Unported License. https://simple.wikipedia.org/wiki/Phase_change
at Simple Wikipedia. Accessed 2022 August 15. Public Domain. https://commons.wikimedia.org/wiki/File:Phase_change_-_en.svg
This document is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
The original LaTeX files and images used to create this reading are
available at:
https://www.chrisspackman.com/educator-resources/readings/states-of-matter/states-of-matter-latex-files.zip
the holding together of atoms by attraction or bonding↩︎
This is a very, very important exception. If ice did not float on water, life may never have evolved!↩︎
Again, solid water (ice) and liquid water are a very important exception. Ice is less dense than water. This is NOT normal.↩︎
collision: (noun) running into each other. The verb form is “collide”.↩︎
This is the same “ion” from “ionic bonds”, but in plasma, the temperature is too high for ionic bonds to form.↩︎
We do not talk in detail about pressure in this reading, just temperature.↩︎